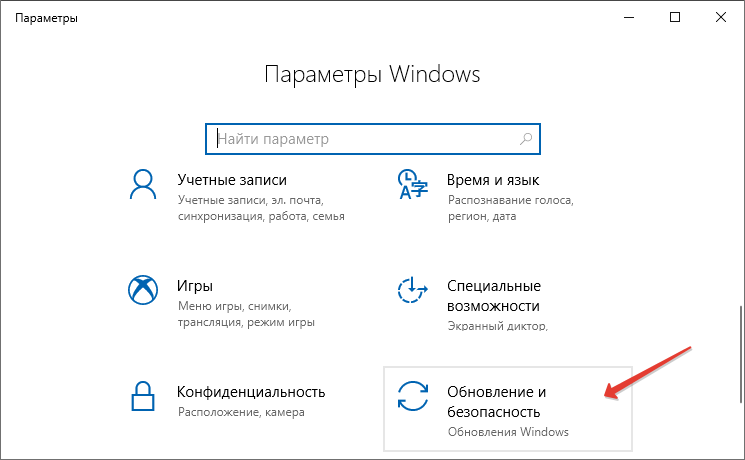
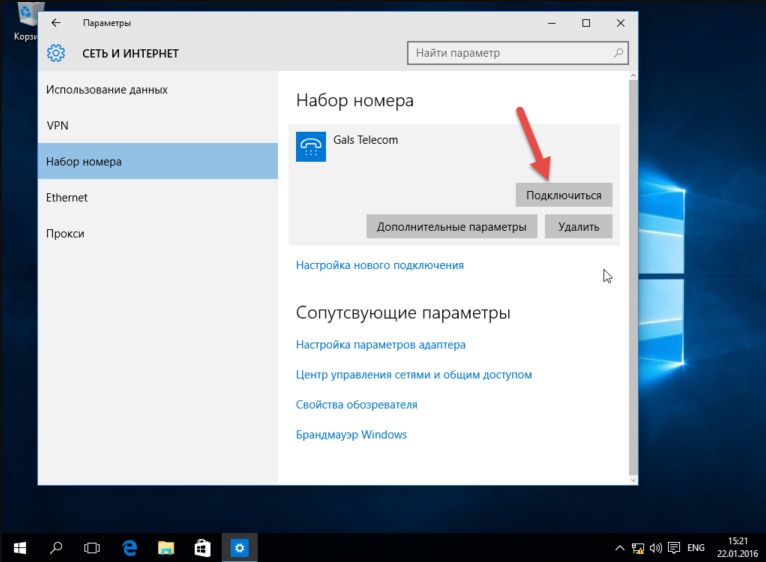
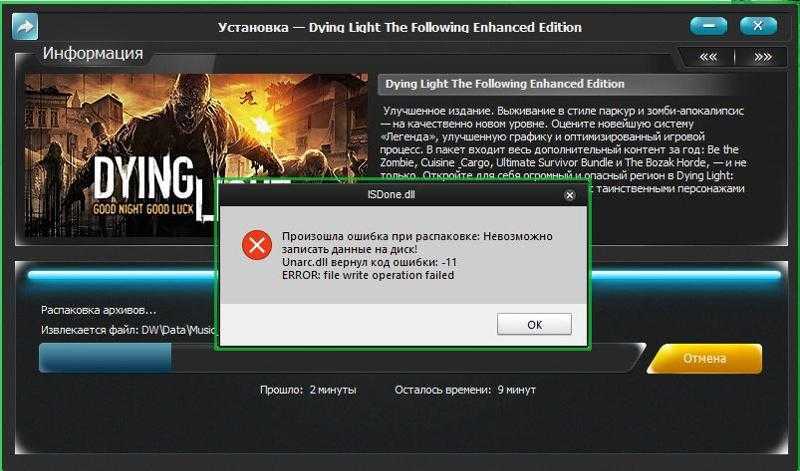
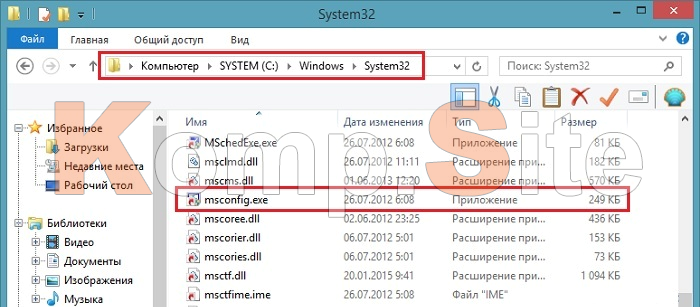
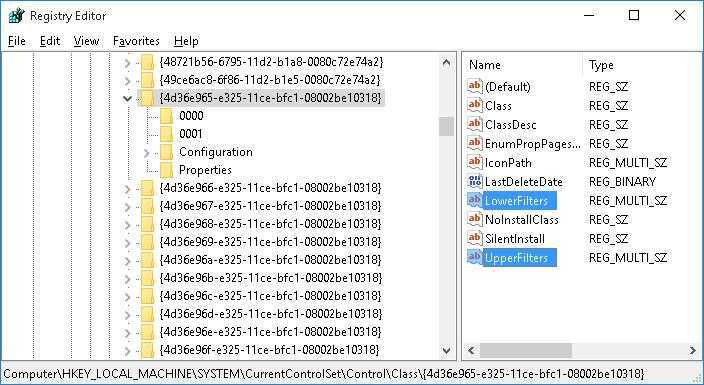
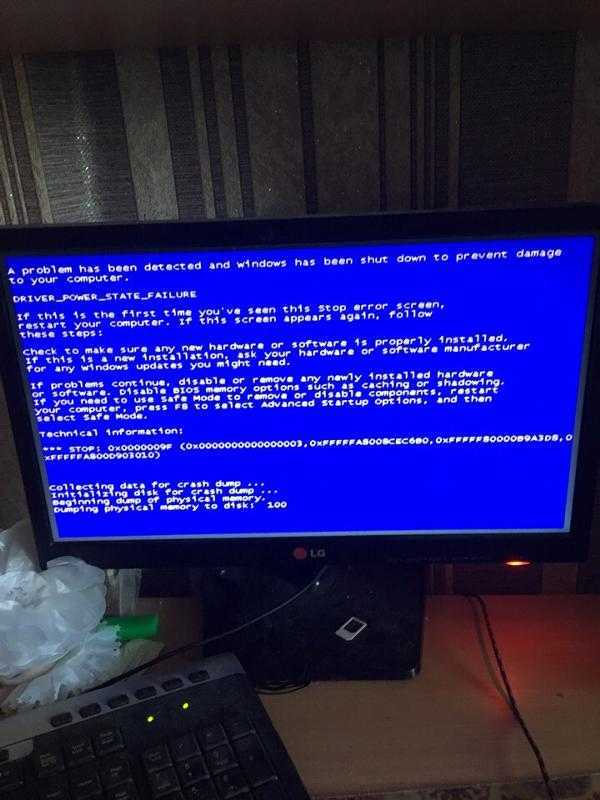
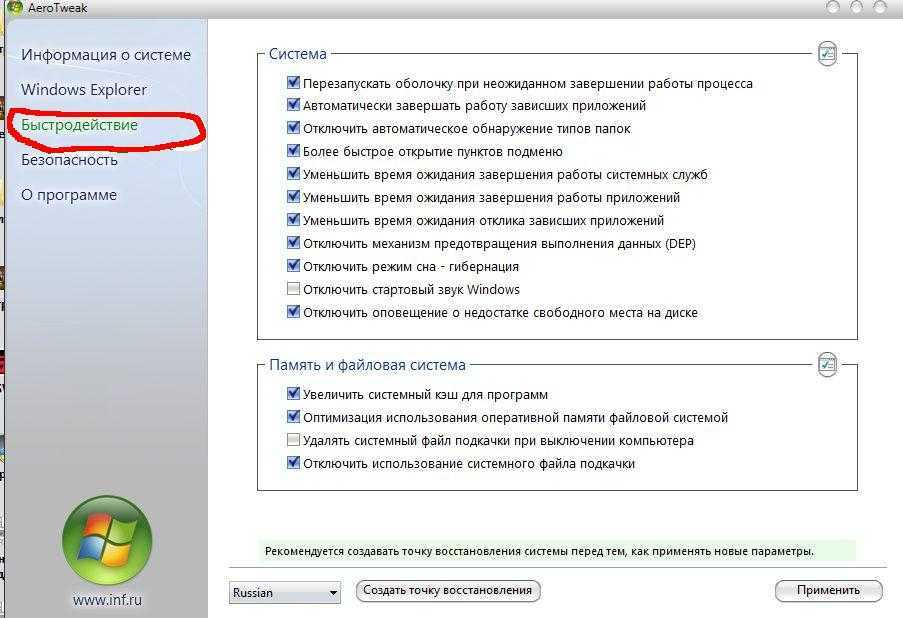
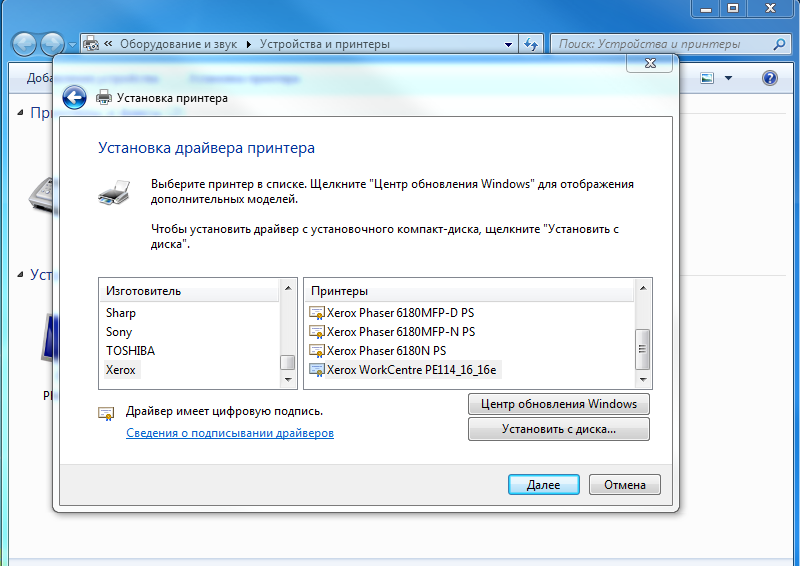
Starting Windows зависает при установке Windows 7 При установке операционной системы Windows 7 может
Читайте в статье, почему средство просмотра фотографий Windows не может открыть это изображение и
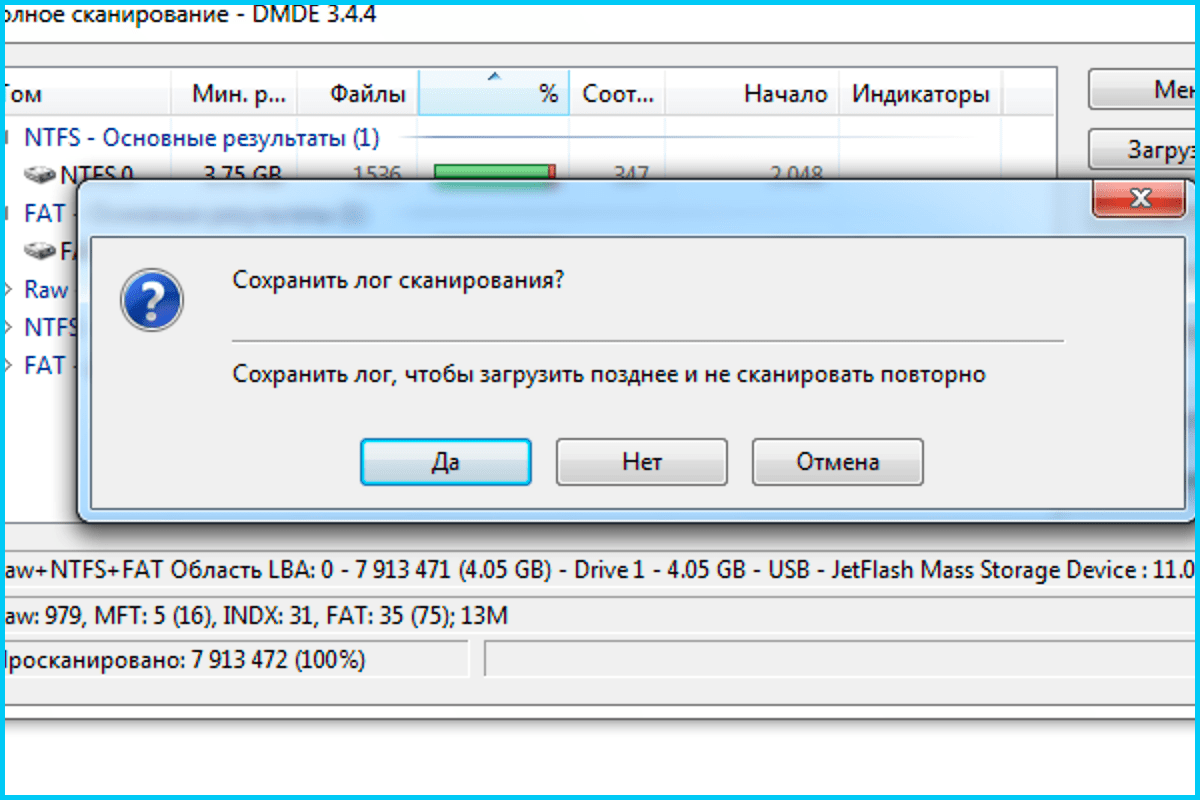
Можно ли исправить диск в файловой системе RAW и восстановить NTFS или FAT32? Как
В статье вы узнаете, что делать, если кнопка «Расширить том» не активна в ОС
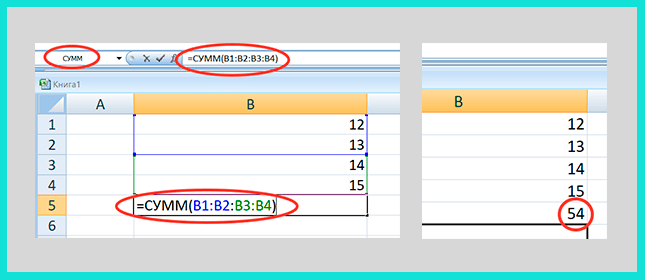
Листы Excel, состоящие из ячеек, сами являются таблицами, но на практике требуются таблицы определенного
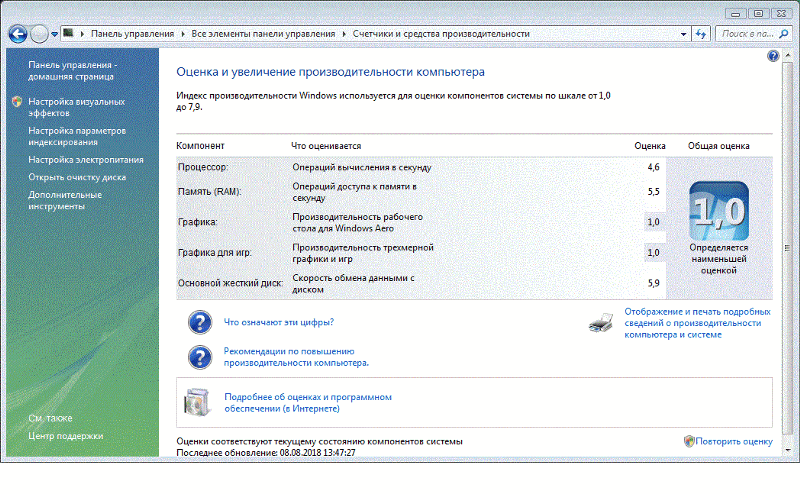
Повысить производительность рабочего стола Windows Aero очень просто, если воспользоваться нашей инструкцией. Всего пара
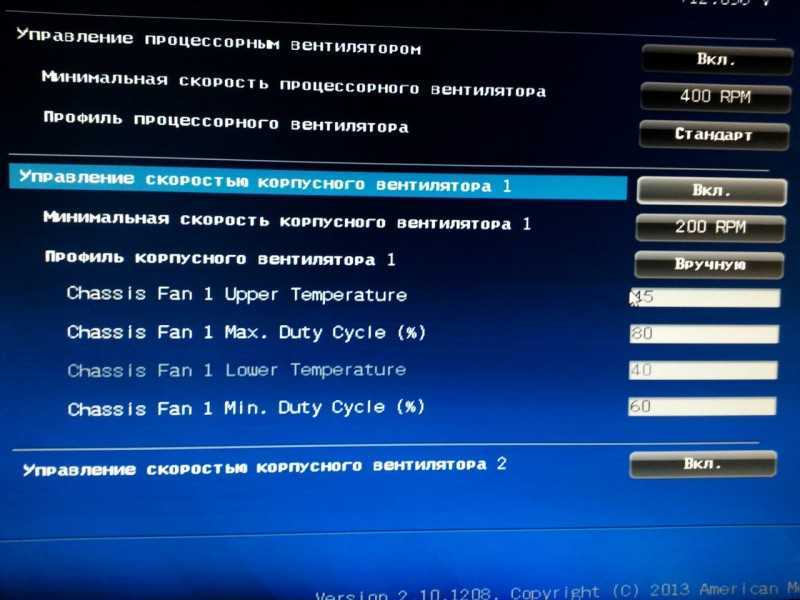
Обзор программ для настройки скорости кулеров на процессоре и других методов, позволяющих увеличить скорость
Хотите знать, что делать, если при загрузке компьютера черный экран? Мы подготовили развернутую инструкцию
Узнай, как сделать Фотоальбом своими руками: идеи оформления и советы начинающим. Скрапбукинг и декупаж.
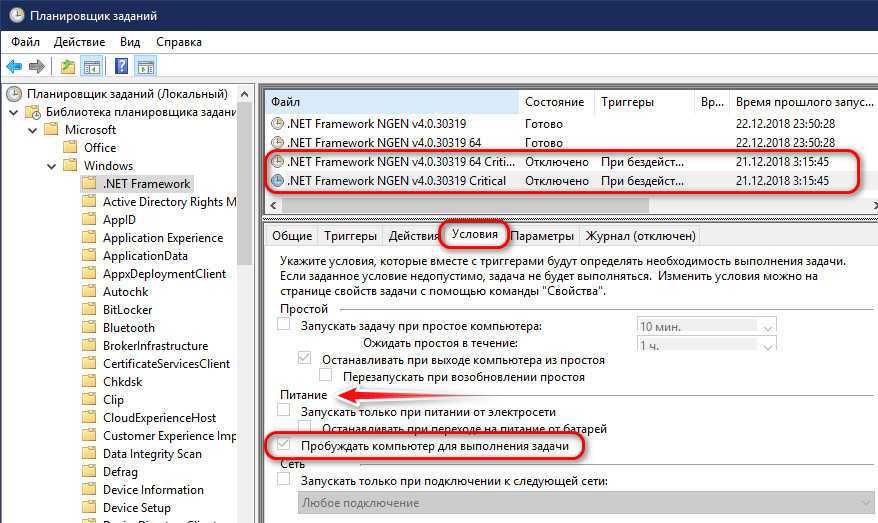
В статье разбираются самые частые причины, почему ОС Windows не всегда может уходить в
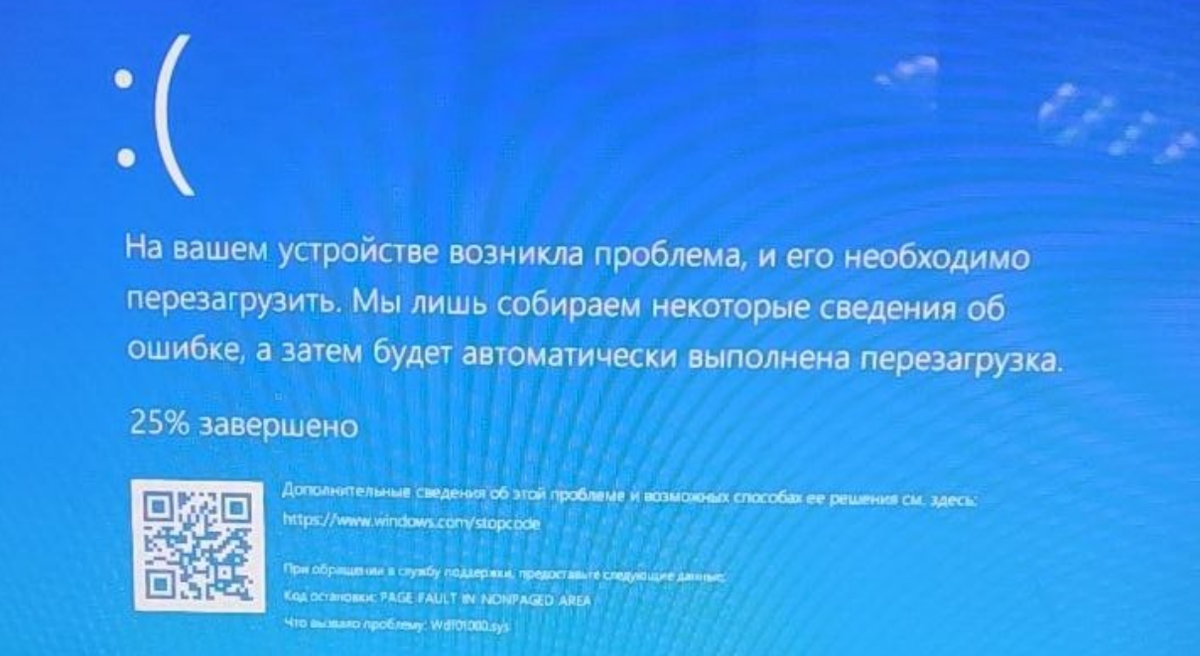
Если возникает ошибка PAGE_FAULT_IN_NONPAGED_AREA Windows 10 (синий экран смерти), то исправить проблему можно просто
Как вывести клавиатуру на экран монитора?
Ошибка пакета windows installer при установке itunes довольно редко встречается, однако решение этой проблемы
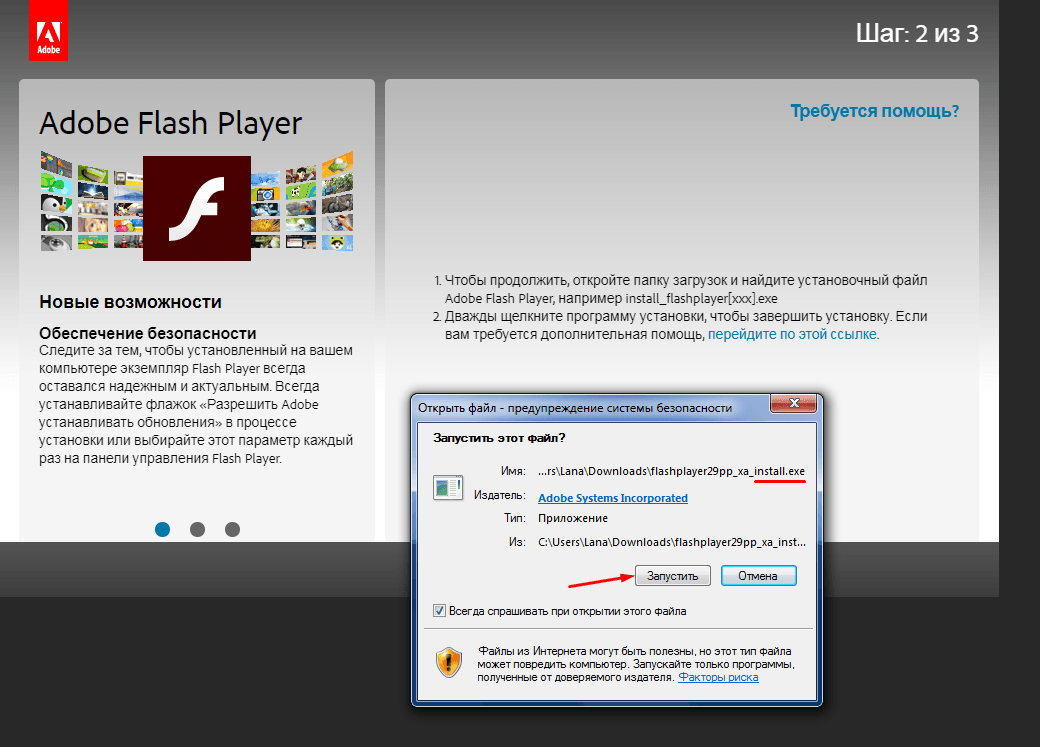
Подробная инструкция о том, как обновить Adobe Flash Player на компьютере или ноутбуке, для
Читайте, как пользоваться Центром уведомлений Windows. Как просмотреть доступные уведомления, очистить их, отключить или
Настройку Windows 10 после установки выполнять нужно всегда. В этой инструкции я даю важные
Как выполняется настройка сети на операционной системе Windows 10. Особенности проводного и беспроводного подключения.
При запуске некоторых программ и игр выскакивает ошибка В системе недостаточно памяти. Сохраните
Многие слышали о ней, хотели бы узнать поближе, но не знают, как зайти в
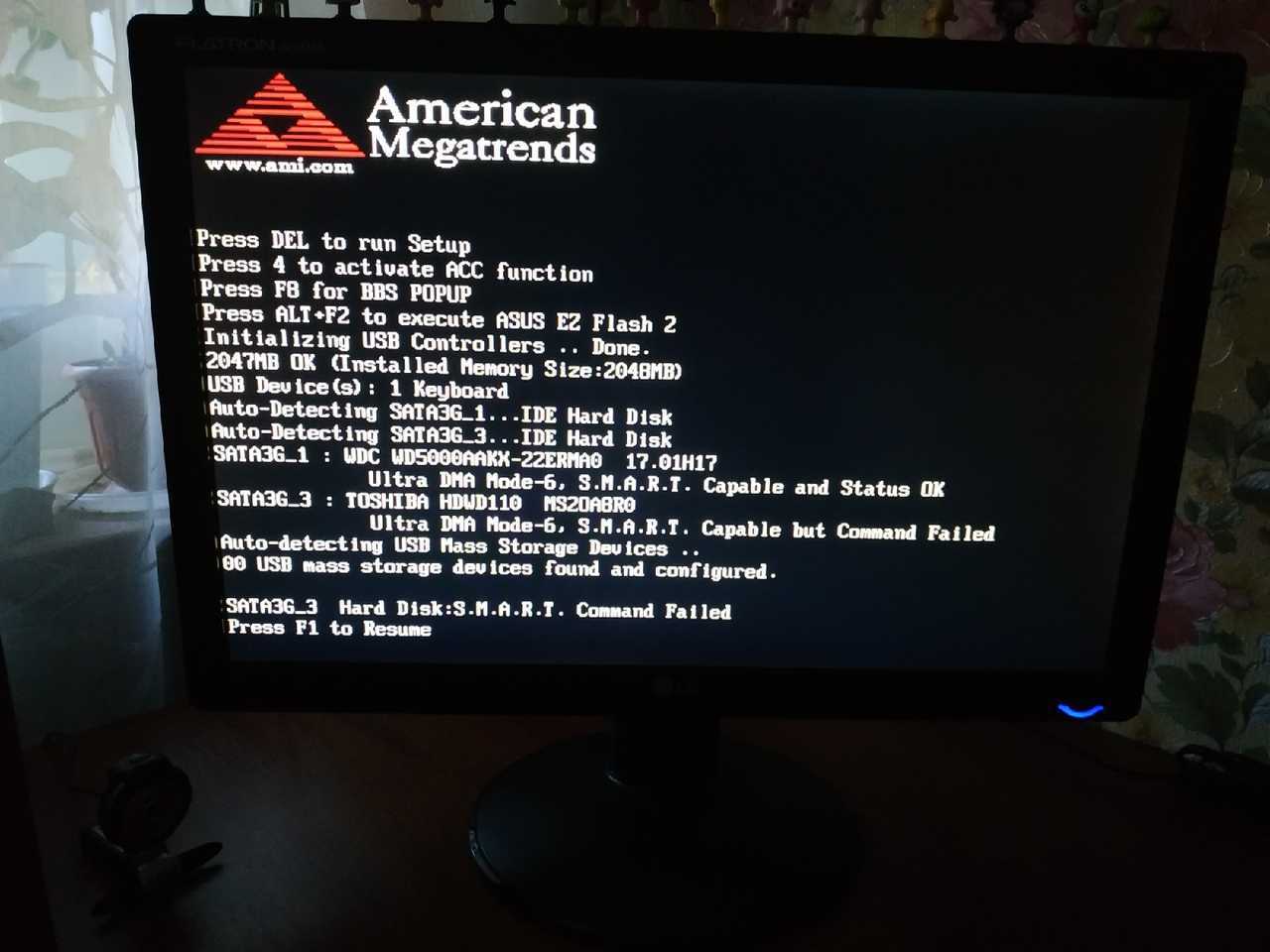
При загрузке компьютера черный экран и надпись «Вне диапазона» Иногда бывает, что при запуске
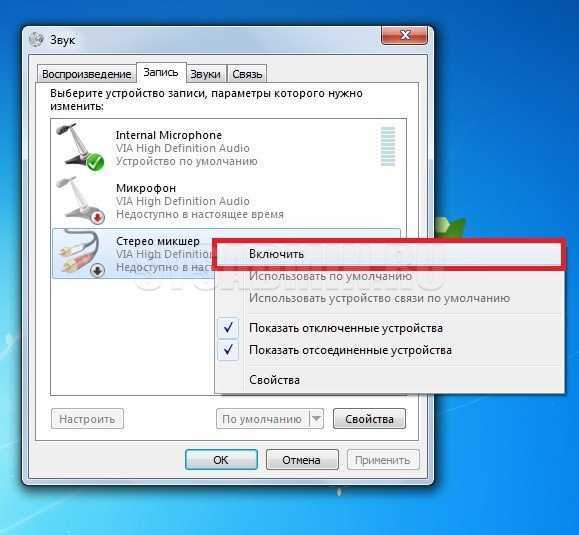
Стерео микшер Windows 10 — описание, функциональные возможности, характеристика. Как включить, пошагово. Что делать,
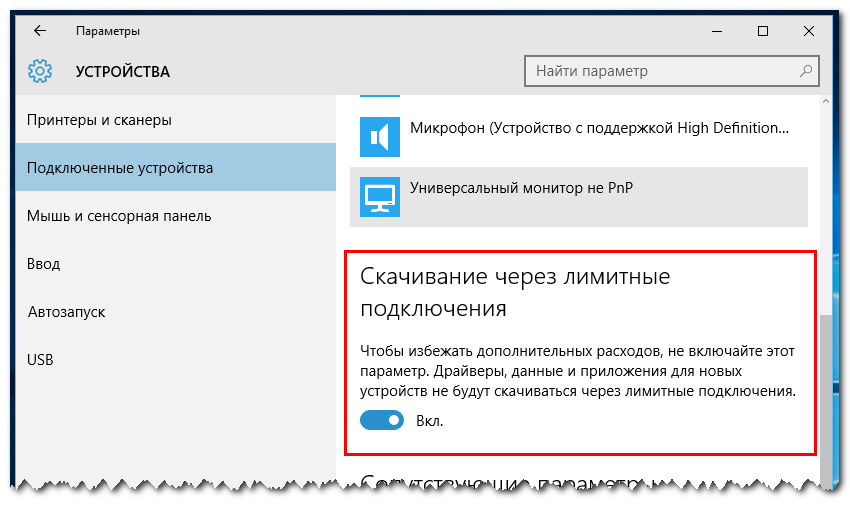
Лимитное подключение - что это и как включить в Windows 10. Подробная инструкция.
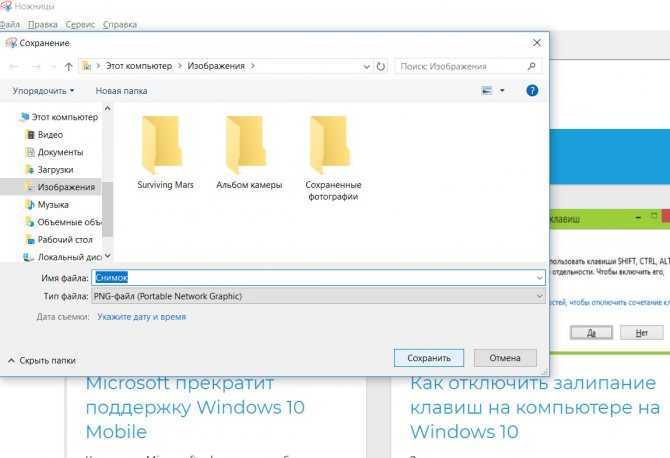
В данной небольшой статье мы расскажем, где сохраняются скриншоты (Print Screen) в операционных системах
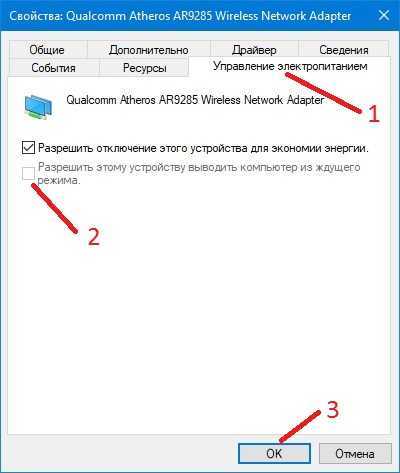
Разбирается полезность спящего режима. Приводятся рекомендации общего характер – работа с драйверами и специальными
Windows 10 не видит DVD привод. Что делать в таких случаях, когда выполнили обновление,
Читайте в статье, почему компьютер долго выключается. Вы найдете эффективные способы по решению данной
Облегчаем себе рутинные задачи при помощи "горячих" клавиш. Полная таблица сочетания клавиш для быстрых
Еще в операционную систему Windows XP разработчики ввели специальную службу, которая отвечала за периодические
Пошаговая инструкция по установке драйверов на Windows 10, Windows 8.1, Windows 7. Драйвера Windows.
Как сделать скриншот экрана на операционной системе Windows 10. Использование встроенных средств ОС, включая

![Программа просмотра фотографий windows не может открыть это изображение [решено на 100%]](http://piter-begom.ru/wp-content/uploads/5/0/5/50541c389a52a7ae5095d80ff60749ac.jpeg)